
|
|
|
|
|
INSIDE THIS ISSUE » Introduction » NTQR Myths and Facts » Especially for Sonographers » Counselors Corner » Coding Connection » Criteria for NT Measurement » NTQR Fast Facts "The NTQR web-based course is outstanding. The content, methodologies, and feedback provided are superb and the information is presented in an organized and interesting manner. I was impressed with the amount and quality of information I was given. For anyone doing NT measurements, the NTQR program is a must." 
Patrick S. Ramsey, M.D., M.S.P.H. ACOG JFCAC Chair Director, CRWH Biorepository Associate Professor - OB/GYN & Public Health University of Alabama at Birmingham |
Introduction The Nuchal Translucency Oversight Committee It is our pleasure to present the first edition of the Nuchal Translucency Quality Review (NTQR) Program Newsletter, the NT Examiner. We intend the newsletter to be a quarterly update of information and activities of the NTQR Program. Our audience is our key stakeholders; providers of NT and first trimester risk assessment as well as laboratories, allied associations and societies, and insurers. The newsletter aims to be informative and perhaps just as importantly, we hope that it will encourage you to let us know what more we can do to expand and enhance the NTQR Program. About NTQR Who We Are The Nuchal Translucency Quality Review Program is a consensus based national NT education and quality review program which is open to ALL providers of NT measurements and first trimester risk assessment. The program was established by recognized leaders in the field of prenatal diagnosis and continues efforts to involve all relevant clinical professional organizations as well as prenatal diagnostic laboratories. The NTQR Program intends to educate providers not only on technical aspects of NT measurements but also to disseminate information on various strategies for optimization of first trimester risk assessment as well as additional risk factors associated with abnormal NT measurements. Program Goals
How to Become NTQR Credentialed
|
||||
|
|

"NTQR values and respects all methods
 |
NTQR Myths and Facts By Larry Platt, MD NTOC Member Prof. Ob-Gyn, Geffen School of Medicine at UCLA, Director, Center for Fetal Medicine and Women's Ultrasound Myth The NTQR was established as a money-making venture for the Society for Maternal Fetal Medicine (SMFM) Fact The NTQR was established by the SMFM when it was recognized that there was a need for a recognized consensus based United States Quality monitoring program for Nuchal Transluceny ,an analyte in the first trimester risk assessment . The Maternal Fetal Medicine Foundation (MFMF) was established as an independent non profit 501 c3 to manage the program. Monitoring fees established are to cover the costs associated with the start up and ongoing costs of the program. No physician on the NTQR receives any remuneration for their activities with the NTQR or MFMF. Each member (physician and non physician members ) has donated hundreds of their discretionary hours to the start up of this program because they believe it is a very important national precedent for medicine. Fees charged will be only be used to cover program costs .There is no plan to generate any profit from this venture. One must remember that even "free" programs have expenses that must be covered by someone. The committee members are doing everything they can to seek alternate sources of funding including grants. We invite your suggestions . Myth NTQR believes that Nasal bone assessment is of no value in first trimester risk assessment. Fact Nasal bone is a very valuable marker which is best used as a secondary test. Myth NTQR does not accept any registrant who has been credentialed by another organization. Fact NTQR values and respects all methods of quality assurance. Myth The place to make the measurement of the nuchal translucency for the NTQR is different than where the measurement is made for Fetal Medicine Foundation (FMF). Fact The measurements are in the exact same place - on the inner part of line with none of the horizontal cross bars protruding into the free space. The NTQR Stance on Nasal Bone Assessment NTQR believes that nasal bone assessment is a valuable additional marker in the assessment of risk for trisomy 21. Nasal bone is a marker that should not be used in isolation but rather as a secondary or contingent test as part of first trimester down syndrome risk assessment. The methods for Nasal bone imaging in the first trimester requires evidence of education and training. The question of ongoing quality assurance has not yet undergone thorough scientific evaluation but in fact may be the method used in the future to maintain competence and consistency of reporting. |
||
|
|
|
|
|
|
Counselor's Corner By Renee Laux, MS NTOC Member NSGC Representative With the advent of new options for first trimester screening, genetic counselors face even more challenges in educating patients about their many choices. Counselors will need to discuss the many options for screening available to the patient and be able to explain the differences in simple terms that patients can understand. Below are several important points to be discussed in a counseling session:
|
  |
|
Further discussion about counseling issues will be in future newsletters including combined, fully integrated, serum integrated, sequential and contingency screening strategies. |
|
|
|
Coding Connection By Dan O'Keeffe, MD NTOC Member Medical Director Phoenix Perinatal Associates. Phoenix Arizona Members of the Nuchal translucency Quality Review Committee, along with the Society for Maternal-Fetal Medicine's Coding Committee, worked for three years to obtain a CPT code for nuchal translucency. Starting January 1, 2007 there are two new CPT codes. 76813 is the CPT code for ultrasound examination for nuchal translucency measurement of the first fetus. 76814 will be the CPT code for ultrasound examination for NT measurment for each fetus after that. These CPT codes can be billed along with a 76801 (first trimester ultrasound) when done for an appropriate indication. Until January 1, 2007, there is a white paper on the Society for Maternal Fetal Medicine's web site, under the coding section, that gives a detailed description of how to bill for nuchal translucency. |
|
Summary of the White Paper on Nuchal Translucency Billing.
A nuchal translucency ultrasound consists of nuchal translucency measurement, a fetal crown rump length measurement and assessment of fetal viability. These are not the components of a 76801 (first trimester ultrasound), therefore a 76801 should not be billed unless you have received permission, in writing, from the health plan to use the 76801 in this manner. In discussions with most major insurance companies in a number of markets it appears that the 76999 code (unlisted ultrasound procedure) is a CPT code that they recommend to be used. This unfortunately will generate additional work because you will have to send in paper claims that go through medical review. If you do not send in the paper claims, there will be denials and delays so you might as well do that upfront. It would also be wise to contact your local payers and make sure they are in agreement with this and if this is in their billing system. If the referring physician requests a first trimester ultrasound in addition to a nuchal translucency, proper billing would be 76801 and then 76999 with a 59 modifier. Again this will only need to occur until January 1, 2007 when the new CPT codes come into effect. |
 |
|
|
|
|
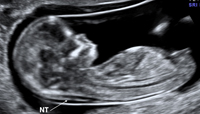
Criteria for Nuchal Translucency (NT) Measurement By Steven L. Warsof, MD Prof. Ob-Gyn, Eastern Virginia Medical School Director, Center for Advanced Fetal Therapy |
|
|
1. Fetal CRL between 38-84mm 2. Margins of NT edges are clear 3. Fetus in Mid Sagittal plane 4. Fetus occupies majority of image |
5. Fetal head in neutral position 6. Fetus observed away from amnion 7. Measurements |

|
| |||||||||||||||||||||||||||