|
|
 | |
The Society for Maternal Fetal Medicine (SMFM) recognized the importance of establishing a nationally respected, consensus-based Nuchal Translucency (NT) quality review program. Approximately two years ago a financially independent (administratively and financially) not-for-profit organization, the Maternal Fetal Medicine Foundation (MFMF) was created by six clinical leaders nominated by the SMFM for their expertise to create and manage the Nuchal Translucency Quality Review program. The current MFMF Board members are Drs. Mary D'Alton, Richard Depp, Roger Newman, Dan O'Keeffe, Larry Platt, and Ronald Wapner.
The first four actions of the MFM Foundation were to:
- establish a NT Oversight Committee to oversee the development of the NTQR program - a nationally recognized, consensus-based education and quality review program that would be responsive to the circumstances and needs of American clinicians and
- develop a business plan including anticipated programs, associated expenses and strategies to pay for the program's activities;
- contract with an experienced data management and website management organization to develop a website that would allow virtually all program activities to be managed electronically; and
- identify and develop a collaborative and responsive relationship with the key participants involved in prenatal screening -
- clinicians (MFMs, Obs, Radiologists, Geneticists);
- non-physician sonographers (SDMS);
- professional societies (ACOG, ACR, AIUM, ACOOG, SDMS & NSGC);
- commercial and private analyte laboratories.
|
|
|
NT CPT Codes (76813 and 76814): Several members of the NTQR and members of the SMFM Coding Committee were instrumental in the creation of two new CPT codes for Nuchal Translucency risk assessment. These new codes are usable as either stand-alone or add-on codes, and will allow practices to properly code and bill for the added work of NT measurements. The codes 76813/76814 include three components: fetal viability, crown/rump measurement and nuchal thickness. These codes are effective on January 1, 2007. They are as follows:
- 76813 Ultrasound, pregnant uterus, real time with image documentation, first trimester fetal nuchal translucency measurement, transabdominal or transvaginal approach; single or first gestation.
- 76814 each additional gestation (List separately in addition to code for primary procedure)
|
 |
|
|
|
The detection of the nasal bone is technically challenging, and as such requires proper training and expertise. If everyone uses the same strict uniform criteria, the outcome of the screening will be optimal, where as individualizing the methods will lead to vastly inaccurate results.
The criteria that NTQR has set forth for assessing the presence or absence of the nasal bone ossification is as follows:
- The fetus must be in a midsagittal plane, similar to the plane required for measuring the nuchal translucency. The facial profile must be well defined and the tip of the nose well seen.
- The image must be magnified sufficiently so that the fetal head and upper torso occupy the majority of the image. This criterion is the same as that required for the nuchal translucency. A second fetus of the same size should not fit in the surrounding space on the image.
- The margins and resolution of the anatomy must be clear and not blurry, so as to see the image detail unambiguously.
- The angle of insonation must be at a 45 degree angle to the fetal profile so as to be perpendicular to the nasal bone. This is necessary to enhance visualization of the nasal bone.
- The echogenicity of the nasal bone must be observed to be equal or greater than the overlying skin on the surface of the nose.
|
Normal Case
 |
Abnormal Case
 |
|
Detecting the presence or absence of nasal bone ossification is difficult and requires practice. There is a learning curve such that sonographers starting their training are far less accurate than those who have been performing the nasal bone assessment for some time. NTQR has suggested that 40 cases is the minimum number required for an experienced sonographer to become proficient in nasal bone assessment. Therefore, NTQR recommends that practitioners and sonographers not start using the nasal bone assessment clinically to determine the risk of aneuploidy until they have performed the evaluation of at least 40 cases and feel comfortable with their technique.
|
|
 |
|
Is it a Myth or a Fact?
By Larry Platt, MD
NTOC Member
Prof. Ob-Gyn, Geffen School of Medicine at UCLA,
Director, Center for Fetal Medicine and Women's Ultrasound
|
|
Is it a Myth or a Fact?
"The NTQR credentialing is only for MFM subspecialists and their sonographers."
It's a MYTH.
NTQR education and credentialing is open to all interested physicians including General Ob/GYN, Radiologists, or other specialists. They may be M.D.'s or D.O.'s. There is separate credentialing for sonographers.
|
 |
|
Is it a Myth or a Fact?
"NTQR will only hold educational courses at the professional society (ACOG, AIUM, SMFM, and SDMS) meetings."
It's a MYTH.
NTQR has already scheduled meetings in conjunction with many other post graduate courses put on by Medical Centers and other CME providers at free standing courses around the country.
Is it a Myth or a Fact?
"NTQR continues to collaborate with principal investigators in the US to provide evidence based studies to analyze best practices."
It's a FACT.
The NTQR is made up of clinicians from across the United States who will be participating in studies to provide evidence based studies to determine best practices.
Is it a Myth or a Fact?
"NTQR has established its complete program and is not amenable to suggestions from individuals who are not members of the NTOC or participants in the NTQR program."
It's a MYTH.
NTQR is a continually evolving program, always interested in hearing suggestion and ideas to improve the program. In fact, we have recently completed our second complete revision of our educational content for our web based and land based courses based on input from participants in these courses.
|
|
The primary goal of the NTQR program is to aid providers in obtaining reproducible and valid NT measurements. The NTQR program provides didactic training, image review (for proficiency testing) and on-going epidemiologic monitoring. In the image review, each provider submits 10 images to an NTQR image review expert to establish proficiency. Once proficiency is established, epidemiologic monitoring techniques are used to assess quality over time. These techniques are cost effective and provide systematic guidelines to determine how each provider's measurements compare to the population at large. Credentialed providers and their supervisors will receive epidemiologic monitoring reports on a quarterly basis, the first quarter reports for 2007 have been distributed.
One of the components of the epidemiologic report is a graphical display containing the providers observed NT measurements as a function of CRL (see Figure 1 below). The solid line in the figure is the population referent, the points are the provider's NT measurements. The example provided in Figure 1, illustrates that the provider is measuring "within expected range" as compared to Figure 2 where the provider is measuring systematically smaller than expected. It is important to note that the observed measurements are not expected to fall along the line, however, approximately half should be above and half below the line over the entire range of CRLs. We also routinely assess the proportion of NT measurements above the 95th percentile and below the 5th percentile.
Figure 1: Provider with NT measurements "within expected range" (measuring similar to the population)
 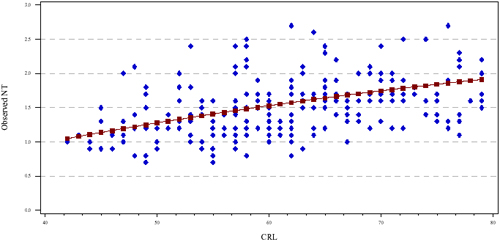
Figure 2: Provider with NT measurements "outside of the expected range" (measuring systematically smaller than the population)
 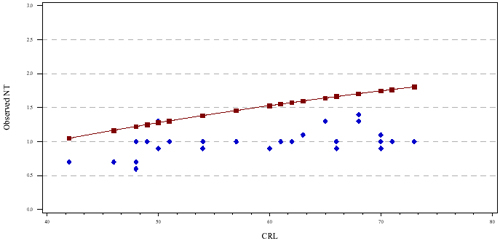
In the next issue of the NT Examiner, we will discuss the statistical methods used to determine whether a provider is within or outside of the expected range and the subsequent steps taken by the NTQR. For further information, please contact [email protected].
|
|
|
|
January 2007 marks our first quarterly distribution of the NTQR program's epidemiologic reports to credentialed sonographers and their credentialed supervisors. Within days we have received our first dividend. A sonographer report that was outside the expected range was reviewed by the appropriate supervising sonologist who quickly communicated the following to the NTQR: "Our internal QA confirms what the report NTQR generated suggested; the sonographer's NTs are all over the map. I would like the sonographer to redo the educational module, retake the test, and resubmit images." The NTQR can respond to that request with educational content, as well as image review and feedback - all quickly done with our web-based program.
The NTQR program's epidemiologic monitoring of NT proficiency offers objective screening of image proficiency and assessment of quality over time for individual providers - but only for NT providers that are NTQR credentialed and who submit a sufficient number of their NT case data to analyte laboratories that cooperate with the NTQR and that in turn exchange NT related data with the NTQR.
The quarterly NTQR Epidemiologic monitoring reports are cost effective and eliminate the need to submit images annually. It's easy for credentialed providers to get started: create a NTQR Provider Practice Account, record the sonologist and sonographer NTQR codes and select clinical data on the lab requisition form for at least 30 cases per year to a lab that participates in the NTQR program. Data for an individual provider can also be aggregated across multiple analyte labs as long as each lab cooperates with the NTQR program.
|
|
Questions related to First trimester screening will present many challenges during genetic counseling sessions. It will be our task to present this evolving information in a coherent fashion so that patients can make appropriate informed decisions. These are some questions that have been submitted to the Genetic Counselor's corner.
Q. My patient wants the screening that, if screen negative, will reassure her the most that the fetus does not have Down syndrome. Timing is not an issue for her as she states that she would not pursue invasive diagnostic testing. What screening would be best?
A. Fully Integrated screening. This includes NT measurement with PAPP-A between 11 and 13 6/7 weeks followed by second trimester biochemical screening (MSAFP, BHCG, uE3, and Inhibin) between 15-20 weeks. After which a single final risk assessment for Down Syndrome would be made.
Q. My patient is undecided about whether she would have CVS or amniocentesis. She would like biochemical and ultrasound screening that would allow for either diagnostic test, if screening increased her risk. What screening would you recommend?
A. Stepwise Sequential Screening. This includes NT measurement with PAPP-A and either free or total BHCG between 11 and 13 6/7 weeks. If screen positive she would have the option of CVS. If she was screen negative, then she would go on to second trimester biochemical screening (MSAFP, BHCG, uE3, and Inhibin) between 15-20 weeks. She would then have the option for amniocentesis at this point.
Q. It was not possible to obtain an NT for my patient at her appointment at 11+3 weeks. She still desires the screening that can reassure her the most. What are her options?
A. Serum integrated screening now, or she can return within 2 weeks to see if an NT can be obtained at this later date. If not, have her blood drawn at that time for serum integrated screening.
Q. My patient desires screening for Down syndrome and would choose to have a CVS but would not choose to have amniocentesis, as she had complication in a previous pregnancy following an amniocentesis. What screening would be best for her?
A. Combined First trimester screening which includes NT measurement with PAPP-A and either free or total BHCG between 11 and 13 6/7 weeks. This would be followed by MSAFP alone in the 2nd trimester to screen for open neural tube and ventral wall defects.
If you have any questions that you would like to submit to future editions of the NT Examiner, please contact Renee Laux at [email protected].
|
 |
|
|
There is much more to performing NT screening for aneuploidy than just doing the sonogram, and obtaining a good quality images. Even if you are new at NT measurements, you know that patients frequently have questions. Even after having a complete genetic counseling session, patients often will ask the sonographer questions that they forget to ask the genetic counselor. Complex questions should always be redirected back to the genetic counselor or a physician. However, many questions can be answered by the sonographer. You should first know your organization's policy regarding answering patient questions. If you are permitted the latitude to answer questions, are you prepared to answer them?
Much of what we do in sonography is based on research, and NT risk assessment is no different. The first step in preparing for patients' questions is to be aware of the research on NT. That means pulling the papers and spending a little time reading. You will be amazed at how much information can be gained from reading the research that went into NT screening.
Access to these papers, including the FASTER (First and Second Trimester Evaluation of Risk) trial papers, are available through PUBMED http://www.ncbi.nlm.nih.gov/entrez/. If you do not have access to PUBMED, free articles are available on the web. One such article entitled "U/S Clinics: Putting the FASTER results into clinical practice" was written by one of the principal investigators of the FASTER trial, and was published in Contemporary Ob/Gyn on Jan 1, 2007. This article is available for free at (http://www.contemporaryobgyn.net/obgyn/article/articleDetail.jsp?id=283484).
Obtaining NT credentialing is the first step in a journey of NT screening. Being familiar with the key studies on NT screening will not only provide a deeper understanding of NT screening, but will also enhance your role as a member of the NT screening team. More importantly, the patient is better served.
| |
|
|
NTQR Finances
By Richard Depp, MD
President, Maternal Fetal Medicine Foundation
Member of the NTOC
Executive VP, Society for Maternal Fetal Medicine
|
1) How does the EZ-Pass Fee Collection work?
The NTOC opted to adopt an EZPass approach to collect monitoring fees. This will work similarly to the method used on many toll roads in the US. The users create and pre-fund, a Practice Account. As labs forward patient data to the NTOC, the account is debited. Once the account falls below a predetermined level, it is automatically replenished. The EZPass approach avoids the necessity of submitting invoices to participating providers, which would result in additional costs to the NTQR as well as to the provider's practice. The fee may be prepaid by check or periodically by Credit card in much the same way as with other periodic fees.
It is necessary for the provider's practice to register a Physician Provider Account (PPA) which is necessary for the linkages to create NTQR's quarterly epidemiologic monitoring reports for NTQR credentialed providers in the practice. It is also necessary to provide a financial linkage and promote a number of efficiencies with regard to consolidation of fees.
In future issues we will discuss other Financial issues such as - what services the fees cover, management of surpluses and deficits and the relative merits of a Member fee versus a Click Fee.
|
|
This will be the first in a series of articles for the NT Examiner to clarify the nine criteria established by the Nuchal Translucency Quality Review (NTQR) Program for NT measurements. It is the goal of the NTQR to standardize the NT measurement. It is critical for First Trimester Risk Assessment that the NT measurement be done uniformly, correctly, and precisely. The NT measurement is unique in diagnostic obstetrical ultrasound as fractions of mm's can make significant differences in individual risk assessment for Down Syndrome, patient's decisions for diagnostic testing, and the overall effectiveness of any Down Syndrome screening program.
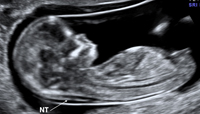
Nuchal Translucency measurements must be between 10 3/7 weeks and 13 6/7 weeks gestation. This is equivalent to a CRL measurement between 38-84 mm. In this window of time CRL accuracy for gestational dating is 3-5 days. The CRL measurement must be included with NT measurement as the NT measurement is converted into multiples of the mean (MOM) for the CRL. The measured CRL is not the actual anatomic CRL but rather the longest straight line measurement of the fetus while its head is maintained in the neutral position. See figure 1. Unlike the NT measurement in which the longest of three good measurements is used, the CRL uses the average from 3 good measurements. NT measurements can be obtained either transabdominally or endovaginally.
- Margins of the NT edges are clear. Although this may seem obvious, it is critical that the margins of the fetal skin edges and facial structures are crisp and clean. Fuzzy images will make measurements inaccurate. The angle of insonation needs to be perpendicular to the NT space. Any misalignment will introduce inaccuracy. In addition the NT lines themselves should be clear. Fuzzy lines will make caliper placement inaccurate.
- Fetus in Midsagital Plane. The NT measurement should only be made in the midsagital view with the fetal spine seen in cervical and thoracic regions. The tip of the nose should be clear in fetal profile. The third and fourth ventricles of the fetal CNS are also frequently seen. Structures not in the mid line should not be seen. If fetal ribs, stomach or heart are seen in the NT image, this would indicate an oblique scan which is not in the midsagital plane and therefore would be an inappropriate image for NT measurement.
|
|
Upcoming Land-based NTQR Courses
- 27th Annual Meeting
The Pregnancy Meeting™
San Francisco Hilton & Towers
San Francisco, CA
Feb 5-10, 2007
http://206.18.123.200/index.cfm?zone=calendar&nav;=meeting
- 2007 AIUM Annual Convention
Marriott Marquis Hotel
New York City, NY
Mar 15-18, 2007
http://www.aium.org/cmeactivities/events/ann2007/intro.asp
- 74th Annual Conference
American College of Osteopathic Obstetricians and Gynecologists
LaQuinta Resort
Palm Springs, CA
March 27-31, 2007
http://www.acoog.com/events.html
- 55th Annual Clinical Meeting
American College of Obstetricians and Gynecologists
San Diego Convention Center
San Diego, CA
May 5-9, 2007
http://www.acog.org/acm/

|
Fetal and Women's Ultrasound 2007
Hyatt Regency Dallas
Dallas, TX
May 18-20, 2007
http://www.iame.com/courses/fetal0507/fetal.html
OBGYN Ultrasound Update
Chicago, IL
Aug 24-26, 2007
http://fmfaus.meetingpro.info/
2007 SDMS Annual Conference
Red Rock Resort, Casino, Spa
Las Vegas, NV
October 11-14, 2007
http://www.sdms.org/meetings/default.asp
IAME 8th Annual Obstetric Ultrasound in the High Risk Patient
The Venetian Resort Hotel
Las Vegas, NV
October 26-28, 2007
http://www.iame.com/courses/hirisk1007/hirisk.html
10th Annual Mid-Atlantic Ultrasound Symposium for Obstetrics and Gynecology and Nuchal Translucency
The Hilton Hotel at Ocean Front
Virginia Beach, VA
November 9-10, 2007
|
|
|
EDITOR-IN-CHIEF
Steven L. Warsof, MD
[email protected]
|
|
LETTERS AND OTHER INQUIRIES
Send letters to the editor and all other inquiries to the NT Examiner
newsletter,
Nuchal Translucency Quality Review 409 12th Street, SW
Washington, DC 20024, or send e-mail to [email protected].
|
|
|
|
|
| | | |
